Hyperthermic intraperitoneal chemotherapy (HIPEC) refers to the precise cyclic perfusion of the abdominal cavity with chemo-drugs in constant temperature and for a certain period of time. It is an auxiliary treatment method for abdominal malignant tumors and is also a good treatment supplement for surgery, radiotherapy and chemotherapy. It has unique efficacy in preventing and treating peritoneal metastasis of abdominal malignant tumors such as gastric cancer, colorectal cancer, ovarian cancer, pseudomyxoma peritonei, peritoneal malignant mesothelioma, liver cancer, cholangiocarcinoma, and pancreatic cancer, as well as concurrent malignant ascites.
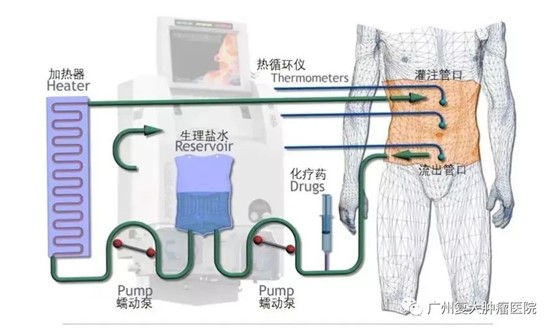
Since HIPEC was first reported by Spratt et al. in 1980, domestic and foreign scholars have continuously explored its technical methods. From a simple direct infusion method after heating the perfusion solution, it has gradually evolved into the current precise intraperitoneal hyperthermic perfusion treatment technology. Its equipment has been continuously innovating and improving, gradually applied in clinical practice. Invited by Fuda Cancer Hospital, Professor Yin Chunzhu from Jilin Cancer Hospital recently shared the mechanism and clinical application of hyperthermic intraperitoneal chemotherapy with us.

Pharmacokinetic advantages of HIPEC
HIPEC is a highly-selective local chemotherapy that has obvious pharmacokinetic advantages compared with systemic intravenous chemotherapy:
Direct abdominal infusion can increase the concentration of chemo drugs in target lesions, leads to slow metabolism and delays drug clearance;
The drug is absorbed through the peritoneum and mesentery and directly flows into the liver through the portal vein system, so it has a killing effect on micro-metastasis in the liver;
The existence of the peritoneal-plasma barrier can delay the absorption of drugs and maintain high drug concentrations in the peritoneal cavity for a long time. Due to the first dose effect of the liver, the amount of chemotherapy drugs entering the systemic circulation is limited, which can reduce the systemic toxic and side effects of the drugs.
The killing effect of hyperthermia on tumor cells
At the molecular level , the thermal effect denatures the proteins on the cancer cell membrane, causing the dysfunction of certain multi-molecular complexes such as receptors, transduction or transcriptases that maintain intracellular homeostasis, and can interfere with proteins, DNA and RNA synthesis;
At the cellular level , warm heat can activate lysosomes, destroy the cytoplasm and nucleus, and directly lead to the death of S- or M-phase cancer cells;
At the tissue level , it can cause embolism of tiny blood vessels in tumors, causing cancer cells to become hypoxic, acidotic or nutrient uptake disorders, which can ultimately lead to tumor cell degeneration and necrosis.
Taking advantage of the above-mentioned difference in heat resistance between tumor tissue and normal tissue, choosing a temperature of 42 to 43°C can kill tumor cells while protecting normal tissue. The combined application of hyperthermia and chemotherapy has a significant synergistic effect, allowing the drug to effectively penetrate into cancer cells.
In addition, continuous cyclic perfusion can mechanically flush and remove the residual cancer cells and peritoneal micro-metastasis lesions in the peritoneal cavity. The flow of liquid during treatment can produce shear force that can directly lead to the death of tumor cells, scouring tissue can also cause anoikis of tumor cells.

During intraoperative and early postoperative intraperitoneal chemotherapy, adhesions have not yet formed in the abdominal cavity, so the drugs can evenly and effectively reach all spaces in the abdominal cavity and fully contact potential lesions. Radical surgery or debulking surgery creates opportunities for chemotherapy to intervene in residual cancer cells in the abdominal cavity. The cancer cells in the patient's body are weak during this period of time, which chemotherapy drugs can achieve better results. However, it is worth noting that the efficacy of HIPEC is also affected by tumor size, treatment temperature, abdominal adhesions, drug dosage and course of treatment.
Hyperthermic intraperitoneal chemotherapy comprehensively utilizes the mechanical lavage effect of local chemotherapy, hyperthermia and large-volume chemotherapy fluid on the abdominal cavity. It has pharmacokinetic and fluid dynamics advantages, and can effectively remove free cancer cells and micro-cancer lesions, and prevent and treat postoperative complications. It is an effective treatment method that is safe, convenient and practical, has few side effects, few complications and can be used repeatedly to treat abdominal recurrence and improve the survival rate and quality of life. Of course, intraperitoneal hyperthermic perfusion is local treatment method. For tumor cells, individualized comprehensive treatment is still needed to better consolidate the clinical efficacy.