Recently, Professor Anna from the University of Rome, Italy, conducted a literature review and meta-analysis on the application of NanoKnife ablation in the treatment of hilar cholangiocarcinoma. This paper provides a detailed analysis of the current application status of NanoKnife for this difficult-to-treat tumor, which we share for your reference and discussion.

Cholangiocarcinoma is classified according to its location into intrahepatic, hilar, and distal cholangiocarcinoma. Among them, hilar cholangiocarcinoma has the highest incidence, accounting for 50% of cases. Clinically, it is divided into Bismuth types 1, 2, 3, and 4, based on the tumor's involvement with the common bile duct and the left and right bile ducts.
Hilar cholangiocarcinoma often causes biliary obstruction and jaundice. Due to the complex anatomy of the hilar region, the resectability rate is low (about 25% of patients are resectable), and the recurrence rate is as high as 50-70%. Most patients are diagnosed with unresectable disease. The standard treatment is palliative chemotherapy (first-line: gemcitabine + cisplatin; second-line: oxaliplatin + 5-FU + folinic acid).
Given the overall lack of effective treatments, various local therapies, including radiofrequency ablation, photodynamic therapy, radiation therapy, and NanoKnife ablation, have been explored for hilar cholangiocarcinoma. NanoKnife, in particular, is promising due to its non-thermal, selective ablation, which may be advantageous in treating hilar cholangiocarcinoma. This paper summarizes and analyzes the current research on this topic.

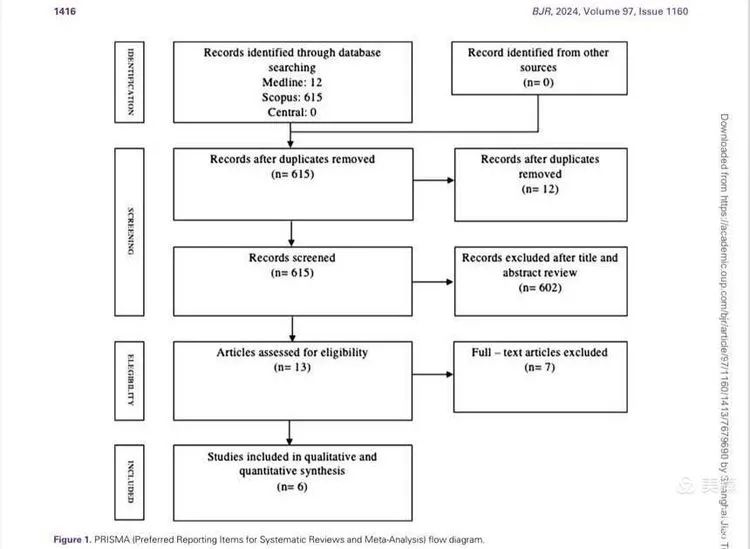
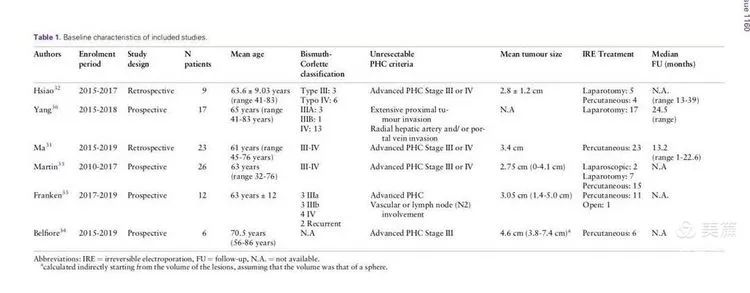
A total of 627 articles were reviewed, and after strict screening, six studies were selected for comprehensive quantitative analysis. The studies included 93 patients, with tumor sizes ranging from 1 to 7 cm. Most patients had undergone chemotherapy. Sixty-one patients received percutaneous NanoKnife ablation, and 32 underwent open NanoKnife ablation. One of the studies is our own research on the combination of NanoKnife and chemotherapy for hilar cholangiocarcinoma, which I will briefly introduce at the end.
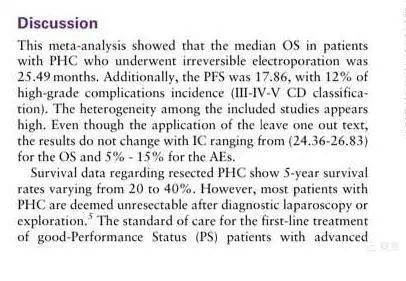
Results: The six included studies reported an average overall survival (OS) of 25.49 months (a significant improvement compared to chemotherapy alone, where survival is around one year), and a progression-free survival (PFS) of 17.86 months. Some patients experienced re-opening of biliary obstructions (30% in our study, 100% in Professor Martin's report). The major complication rate was 12% (with significant variability among the included cases), including biliary infections, bleeding, portal vein thrombosis, biliary fistulas, arrhythmias, and pneumothorax.
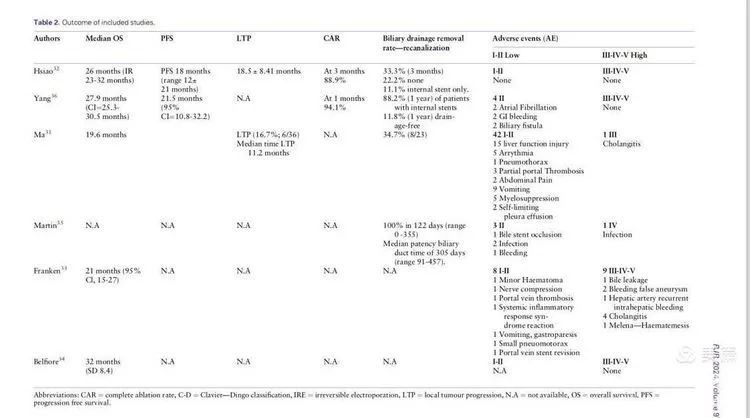
Discussion: Previous studies have shown an average survival of 29.6 months for surgically treated cholangiocarcinoma, 12.2 months for chemotherapy alone, and 2.9 months for best supportive care. This study shows that the combination of NanoKnife and chemotherapy results in an average survival of 25 months, indicating that NanoKnife provides significant survival benefits for unresectable hilar cholangiocarcinoma. However, current research is still very limited due to small sample sizes, non-prospective studies, varying tumor sizes, different numbers of ablation needles used, differences in surgical methods, and inconsistent chemotherapy regimens and timing. Despite these limitations, NanoKnife significantly improves survival in patients with unresectable hilar cholangiocarcinoma.
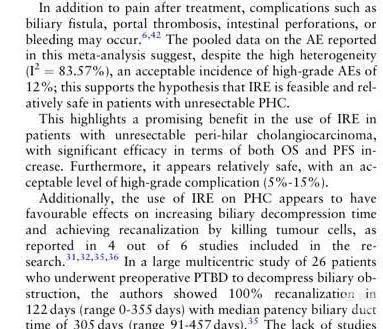
NanoKnife is a non-thermal selective ablation technique that minimizes damage to important structures in the hilar region, particularly blood vessels and bile ducts, making it superior to other ablation techniques. The main complications, including biliary fistulas, thrombosis, perforation, and bleeding, occurred in 12% of cases, which is relatively safe. Furthermore, a multi-center study led by Martin reported a 100% biliary re-opening rate, significantly improving patients' quality of life.
Conclusion: This meta-analysis shows that NanoKnife is a safe and effective treatment for unresectable cholangiocarcinoma. However, due to the limitations of this study, further prospective randomized studies are needed to clarify the role of NanoKnife in the treatment of hilar cholangiocarcinoma.

Our meta-analysis also cited one of our studies on the combined use of NanoKnife and chemotherapy for hilar cholangiocarcinoma, which was the first to report on this combination therapy internationally. This treatment not only leverages the tumor-ablating effects of NanoKnife but also harnesses its electrochemical and immunological effects, making it particularly synergistic for hilar cholangiocarcinoma, a disease that is difficult to treat with conventional ablation. This approach is similar in principle to concurrent chemoradiotherapy and has shown better efficacy and safety.

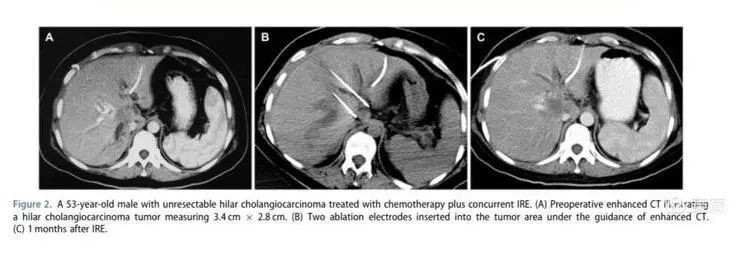
The case we presented involved NanoKnife ablation with parallel placement of two needles. Post-treatment, the tumor showed no signs of activity. The application of NanoKnife for unresectable hilar cholangiocarcinoma holds great potential.